Technology
Patients diagnosed with some types of cancers may be advised additional testing of tumor tissue sample to determine the status of biomarkers including ER, PR, HER2 (ERBB2), EGFR and PDL1. The positive status of these biomarkers forms the basis for selection of targeted anticancer agents which are known to be more effective against cancer.
Few Biomarkers and Targeted Anti-cancer Treatment
| Target | Drug | Cancer Type |
|---|---|---|
| PD L1 | Pembrolizumab | Bladder, Breast, Cervical, Lung, Gastric, Esophageal, Vulvar, Head and neck cancers |
| ER / PR | Megestrol acetate | Breast, Uterine cancers,
Uterine sarcoma |
| HER2 / ERBB2 | Trastuzumab | Breast, Colorectal, Gastric,
Esophageal, Uterine cancers |
| EGFR | Panitumumab | Colorectal cancer |
The tumor tissue sample required for these tests is obtained either by a surgery or via a biopsy; these ‘invasive procedures‘ need an incision (cut) to be made on the skin using a scalpel to take a sample of the suspicious tissue from within the body.
Tissue sampling is associated with risks such as pain, soreness, bleeding and infection. Sometimes, tissue sampling may not be possible because of the above risks. In some cases, insufficient tissue sample may lead to a dilemma due to inconclusive findings. In cases where these problems are encountered, a repeat tissue sampling procedure may be considered but may not always be possible.
It is hence imperative to have a safer and robust alternative to tissue sampling-based methods, which can be relied upon to provide the status of the above biomarkers in order to support a treatment decision in cases where there are challenges to tissue sample-based evaluations.
About Pinaka™
Pinaka™ is an advanced non-invasive blood test which provides therapeutically relevant information via a safe, simple and quick blood draw. Pinaka™ evaluates blood sample to detect ‘Circulating Tumor Cells’ (CTCs), which are cancer cells that escape from the tumor and enter the blood stream. Pinaka™ enriches these CTCs from the blood sample and confirms their identity via a process called ‘Immunocytochemistry’ (ICC). Pinaka also determines the status of the above biomarkers on CTCs either via ICC or by fluorescent in situ hybridization (FISH).
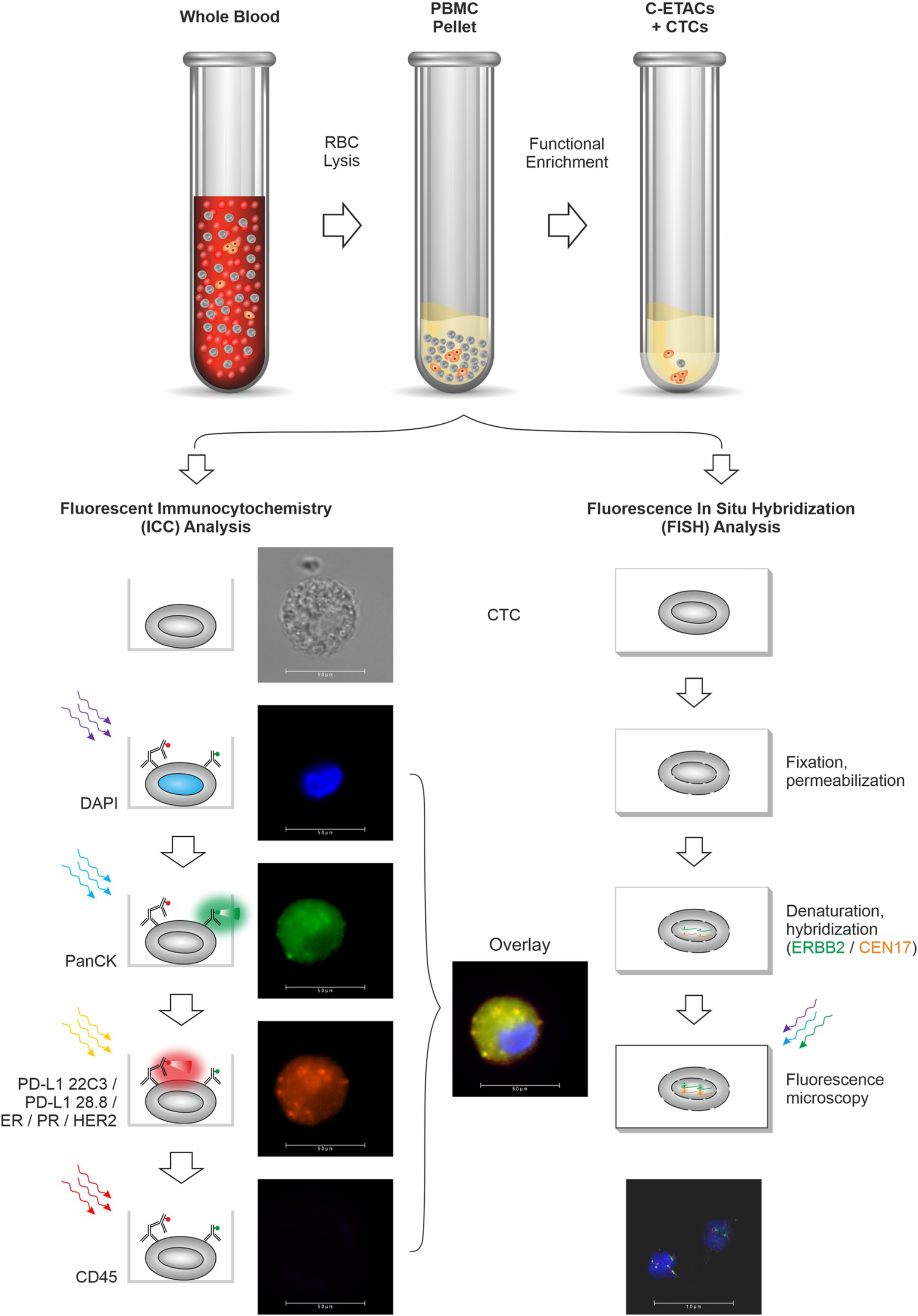
Reliable Technology
Advantages
The technology of Pinaka™ is based on the ubiquity of CTCs and the highly efficient CTC detection process. This technology has a low false negative rate (high ‘sensitivity’) as well as a low false positive rate (high ‘specificity’). These factors translate into a safe blood test for accurate and reliable determination of the status of the above biomarkers.
Turn Around Time
Typically, we are able to complete the test and analysis in 08 days and issue the final report within 10 days of the receipt of the sample at our facility.
Cancer and Biomarker Coverage
| Cancer Type | PD L1* | ER* | PR* | HER2** | EGFR** |
|---|---|---|---|---|---|
| Breast | ✓ 1 | ✓ | ✓ | ✓ | - |
| Endometrial | - | ✓ | ✓ | ✓ | - |
| Esophageal | ✓ | - | - | ✓ | - |
| Gastric | ✓ | - | - | ✓ | - |
| Central Nervous System | - | - | - | - | - |
| Colorectal | - | - | - | ✓ | ✓ |
| Uterine | - | ✓ | ✓ | - | - |
| Bladder2 | ✓ | - | - | - | - |
| Cervical | ✓ | - | - | - | - |
| Head and Neck | ✓ | - | - | - | - |
| Lung3 | ✓ | - | - | - | - |
| Ovarian | - | ✓ | - | - | - |
| Vulvar | ✓ | - | - | - | - |
Pinaka™can provide accurate and reliable determination of the status of the above biomarkers, which can assist physicians to make life-saving treatment and clinical management decisions more effectively.